Indications and Usage

SPRIX® (ketorolac tromethamine) is indicated in adult patients for the short term (up to 5 days) management of moderate to
moderately severe pain that requires analgesia at the opioid level.
- Sprix is not for use in pediatric patients less than 2 years of age.
DOSAGE PRESCRIBING
Dosing and Administration1
For adult patients <65, ≥110 lbs, and normal kidney function:
SPRIX Nasal Spray
1 Box (5 single-day bottles)*
Sig: 1 spray in EACH nostril
every 6-8 hours as needed
Max 4 doses per day, for up to 5 days
For adult patients ≥65, <110 lbs, or with renal impairment:
SPRIX Nasal Spray
1 Box (5 single-day bottles)*
Sig: 1 spray in ONLY ONE nostril
every 6-8 hours as needed
Max 4 doses per day, for up to 5 days
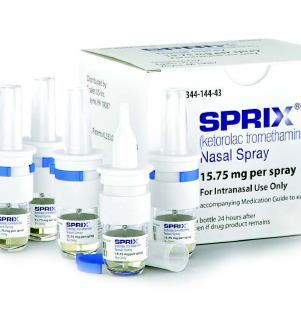
SPRIX is provided
in a 5-bottle carton. Each bottle contains 8 precisely metered doses.

SPRIX can be used:
- Every 6-8 hours
- Up to 4 times per day
- For up to 5 days
SPRIX is not an inhaled product; patients should hold their breath during administration.1
*Discard used SPRIX bottle after 24 hours
- The total duration of use of SPRIX® alone or sequentially with other formulations of ketorolac (IM/IV or oral) must not exceed 5 days because of the potential for increasing the frequency and severity of adverse reactions associated with the recommended doses
- Do not use SPRIX concomitantly with other formulations of ketorolac or other NSAIDs
STORAGE1

- Protect from light and freezing
- Store unopened SPRIX between 2°C to 8°C (36°F to 46°F)
- During use, keep containers of SPRIX Nasal Spray at controlled room temperature, between 15°C to 30°C (59°F to 86°F), out of direct sunlight
- Bottles of SPRIX should be discarded within 24 hours of priming
DIRECT SAVINGS, DIRECT TO YOUR PATIENTS
SPRIX® (ketorolac tromethamine) DIRECT provides your patients with their medication EXCLUSIVELY through SPRIX DIRECT
SPRIX DIRECT offers support for your patients and your practice
Support for Patients
- Automatic $0 co-pay offer for eligible patients with commercial insurance
- Quick home delivery of SPRIX with instructions for use
Support for Your Practice
- Payer claim adjudication
- Registered pharmacist available for
24-hour pharmacy support - Prior authorization assistance
SPRIX DIRECT
Prescribe to Scripts Rx
for no copay card required
Scripts Rx (formerly Mall Pharmacy)
1815 S. Meyers Road, Suite 100
Oakbrook Terrace, IL 60181
NCPDP/NABP: 1468481
NPI: 110 498 2230
Questions, call: (844) 977-7749
Fax: (844) 794-7275