Indications and Usage

SPRIX® (ketorolac tromethamine) is indicated in adult patients for the short term (up to 5 days) management of moderate to
moderately severe pain that requires analgesia at the opioid level.
- Sprix is not for use in pediatric patients less than 2 years of age.
RAPID ABSORPTION
SPRIX® (ketorolac tromethamine) is rapidly absorbed through the nasal mucosa1
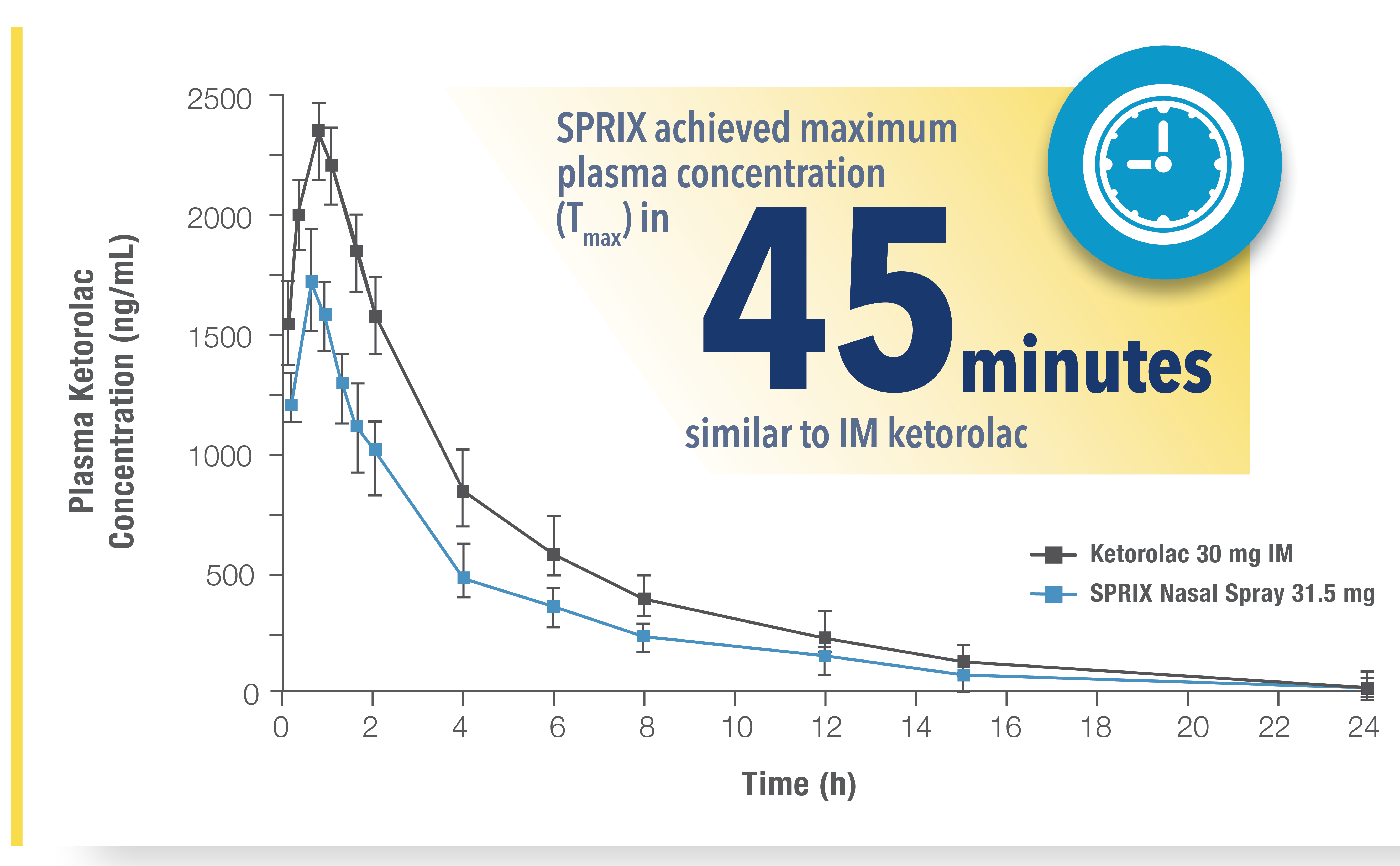
A phase 1, single-dose, 5-way crossover, randomized study (N=15) consisting of 5 periods of identical design, differing only in the allocate treatment and examination of the nares. In all periods, serial blood samples were taken up to 24 hours postdose.1
SPRIX has been studied in patients with allergic rhinitis before and after administration of fluticasone and oxymetazoline. Neither medicine had any clinically significant effect on the PK characteristics of SPRIX.2
Adapted with permission from McAleer et al.
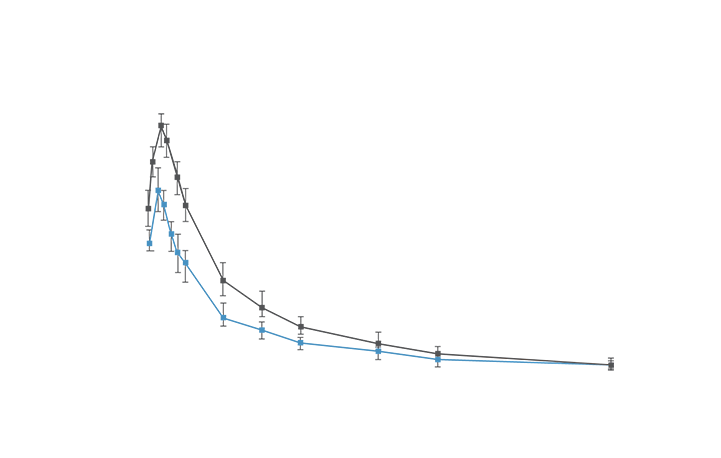
LESS pain
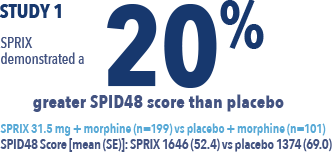
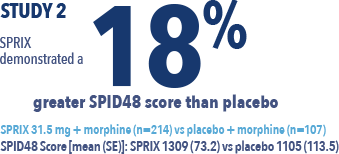
The efficacy of SPRIX® (ketorolac tromethamine) Nasal Spray was demonstrated by a statistically significant reduction in pain vs placebo in an acute pain study of post-abdominal or -orthopedic surgery patients and in a second study of post-abdominal surgery patients2,3,4,5
SPID48 = Summed Pain Intensity Difference over 48 hours
View Study 1 - Brown
A phase 3, randomized, double-blind, placebo-controlled study to evaluate the analgesic efficacy and tolerability of single- and multiple-dose SPRIX in major abdominal and orthopedic surgery patients remaining in hospital for 2–5 days.
Click to View More
View Study 2 - Singla
A phase 3, randomized, double-blind, placebo-controlled study to evaluate the analgesic efficacy and tolerability of SPRIX use after abdominal surgery for up to 5 days.
Click to View More
LESS opioids
Postoperative patients using SPRIX required less morphine vs the placebo group2,3,4
*All patients had access to morphine sulfate (MS) by patient-controlled analgesia (PCA) beginning on Day 0.
View Study 1 - Brown
A phase 3, randomized, double-blind, placebo-controlled study to evaluate the analgesic efficacy and tolerability of single- and multiple-dose SPRIX in major abdominal and orthopedic surgery patients remaining in hospital for 2–5 days.
Click to View More
View Study 2 - Singla
A phase 3, randomized, double-blind, placebo-controlled study to evaluate the analgesic efficacy and tolerability of SPRIX use after abdominal surgery for up to 5 days.
Click to View More
SAFETY DATA
SPRIX® (ketorolac tromethamine) was studied in patients enrolled in two placebo-controlled efficacy studies of acute pain following major surgery
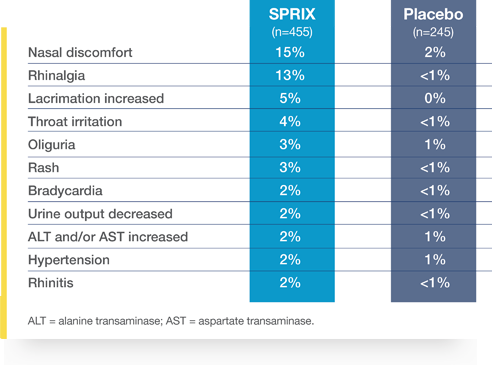
Postoperative patients with adverse reactions observed at a rate of 2% or greater and at least twice the incidence of the placebo group
In clinical studies, 1.5% of patients treated with SPRIX experienced serious adverse events of bleeding or hematoma at the operative site vs 0.4% treated with placebo (hematoma).
The most frequently reported adverse reactions were related to local symptoms, i.e., nasal discomfort or irritation. These reactions were generally mild and transient in nature.
The total duration of use of SPRIX or any other ketorolac formulation should not exceed 5 days.